
Photo (on the left) courtesy of Eran Ittah and McGill iGEM 2023, and photo (on the right) courtesy of Natalie Reznikov and Stephanie Bessette.
We acknowledge the Facility for Electron Microscopy Research at McGill. These photos were taken using the Hitachi Ethos NX5000 dual beam microscope.
Yeast Saccharomyces cerevisiae has become a workhorse in biotechnology due to its robustness and versatility. With a well-studied genome and metabolism, yeast combine features of the eukaryotic kingdom that support processes specific to higher organisms, with the simplicity of manipulation conferred by its unicellular makeup. Well-established genetic manipulation, fast growth rate, and the ability to be cultivated from simple nutrients combined with high-throughput emerging tools make possible the integration of multiple genome modifications into yeast cells in a short time. Moreover, pathways of central metabolism are conserved and partially shared in yeast and plants enabling the reconstruction of terpenoid pathways into yeast’s metabolism. We aim to introduce advances of increasing complexity in engineering yeast as efficient production platforms that tackle global interaction networks from genetic to metabolic level, mimic natural production systems from archaea to higher eukaryotes and engineering of cellular microbioreactors for synthesis of complex, designer compounds.
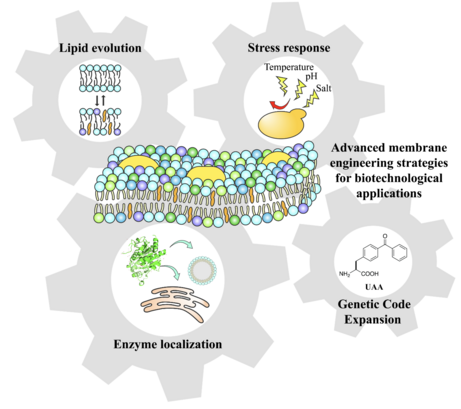
Current approaches mainly address the role of biological membranes as the primary target of the toxic effects of compounds produced in heterologous hosts. In reverse, there is little understanding of, or ability to predict, how differences in membrane composition of the host versus native membranes affect functional capacity of heterologous biosynthetic enzymes and interfere with intracellular trafficking of metabolites and their export outside of the cell. Our group will directly address these aspects by manipulation of the host membrane composition to mimic that of producer organisms or by engineering non-natural yeast membranes. Can we reprogram the yeast membrane composition to enable regulation of biosynthetic pathways occurring in native organisms and trigger formation of membrane-bond metabolic complexes? Can we redirect the synthesis and exchange of lipids between membranes to mimic specific processes in higher organisms (e.g. secretion, lipid droplets formation)? To address these questions, we employ a systematic synthetic biology approach to control the properties and structure of lipid membrane for applications in biotechnology.
Specialized metabolism in plants and microbes is a process of remarkable genetic and biochemical plasticity leading to astonishing diversity of chemical structures that have found broad applications. Yet, accessing new compounds with potent activities for drug discovery is extraordinarily difficult. Introduction of genomic and transcriptomic studies have led to discovery of several biosynthetic pathways of important natural products. However, this process still stands on mechanistic knowledge and very low throughput approaches. We endeavor to introduce artificial intelligence (AI) in specialized metabolism research and to develop AI-assisted standardized Synthetic Biology platforms to accelerate the discovery and reconstruction of biosynthetic pathways in relation to a structure-function of choice.
The biosynthetic enzymes involved in terpenoid biosynthesis are sophisticated biocatalysts responsible for the astounding diversity of chemical structures produced. In native organisms, these enzymes are evolutionary tuned to catalyze specific reactions in biosynthetic pathways governed by a complex regulation and dictate the primary end-product of a biosynthetic pathway without inhibiting normal cellular processes. However, the reconstruction of heterologous pathways in yeast stands on the consumption of endogenous metabolites involved in the native metabolism. Specifically, the production of terpenoids is based on isoprene units produced by a central pathway, sterol biosynthesis and three biosynthesis modules of increased diversification to reach the complexity found in nature. We apply protein engineering strategies to improve catalytic activity, substrate selectivity and product specificity of target enzymes to unlock, control and repurpose the enzymatic potential of target pathways.

Current lack of understanding of the complete biosynthesis of important high-value products remains a major impediment for sustainable production of terpenoids in heterologous hosts. Current projects in our lab focus on:
a. Enzyme discovery or engineering of surrogate activities for reconstruction of missing steps.
b. Optimization of pathways imported into microbial hosts to mimic the efficiency and fine regulation of natural hosts (e.g. optimal catalytic activities, rapid conversion of labile or toxic intermediates and the control and coordination of multifunctional enzymes or shared metabolites).
c. Synthesize enough amounts of target compounds, derivative libraries or novel chemicals for bioactivity screening.